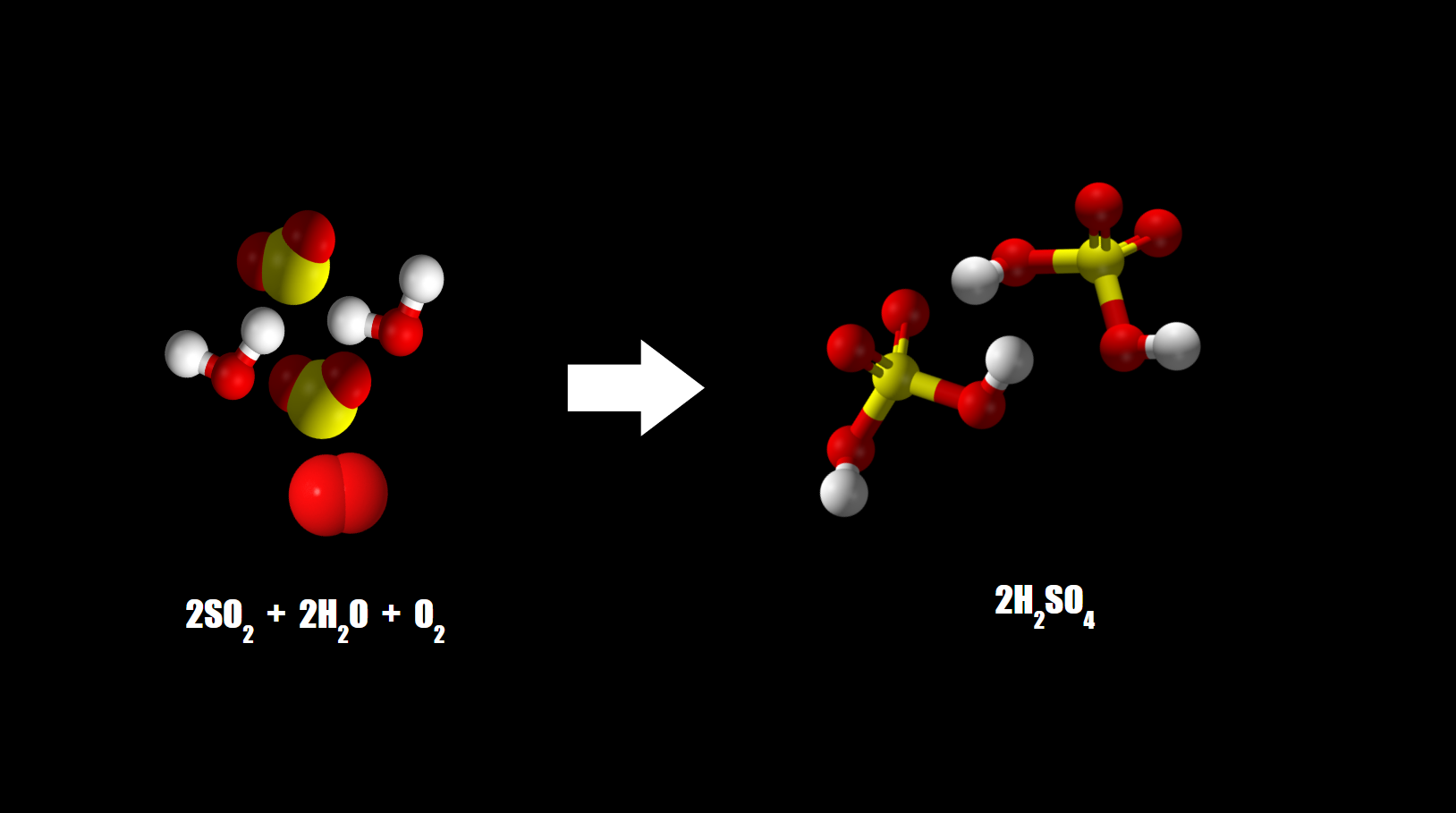
Sulfuric acid rain
Sulfur dioxide is spontaneously oxidized to sulfate as it is captured by water droplets in the presence of oxygen, forming sulfuric acid. Sulfate can be used as a terminal electron acceptor in cell respiration.
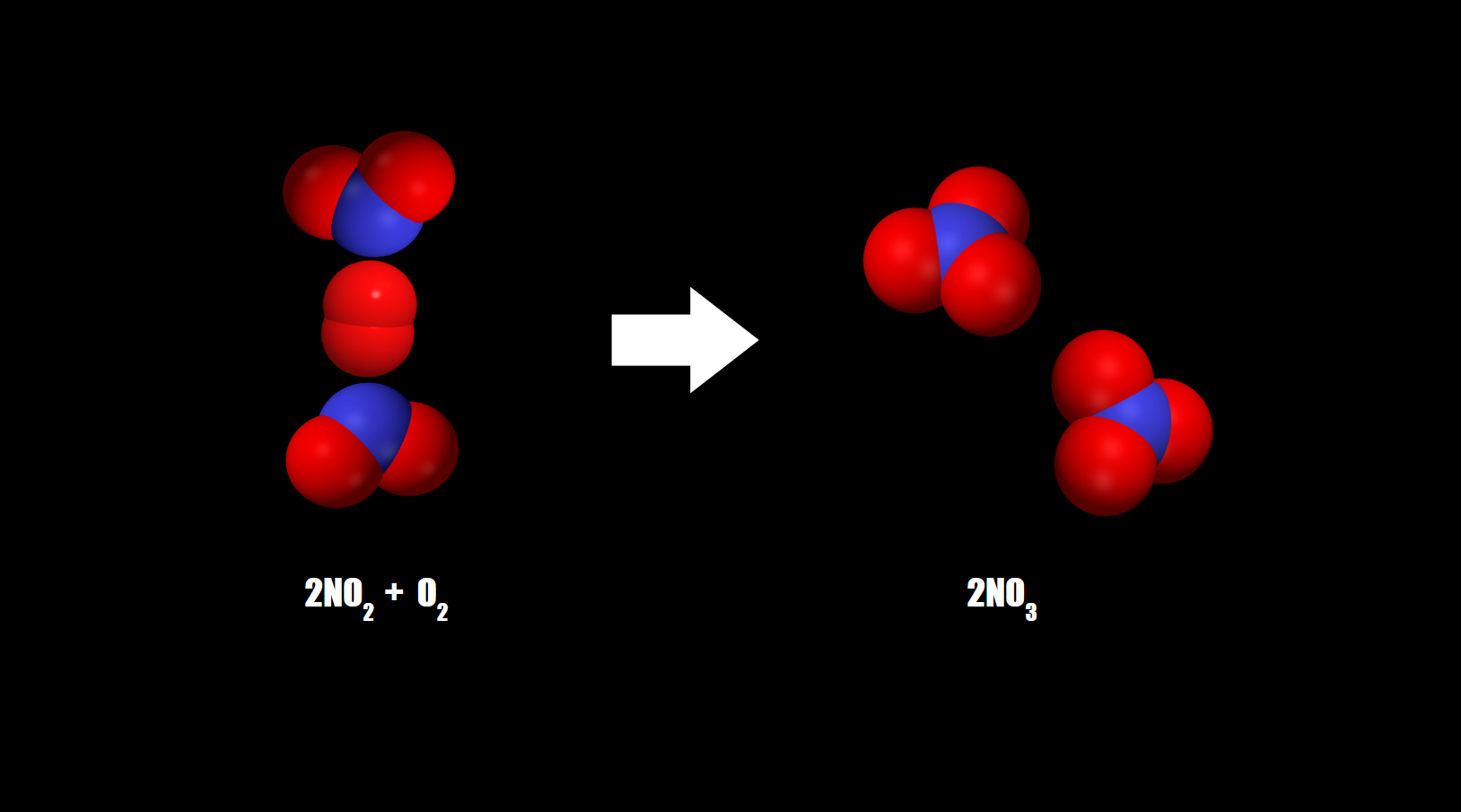
Nitrate
Nitrite is consumed and converted into nitrate by nitrifying microbes. Nitrate can be used as a terminal electron acceptor.
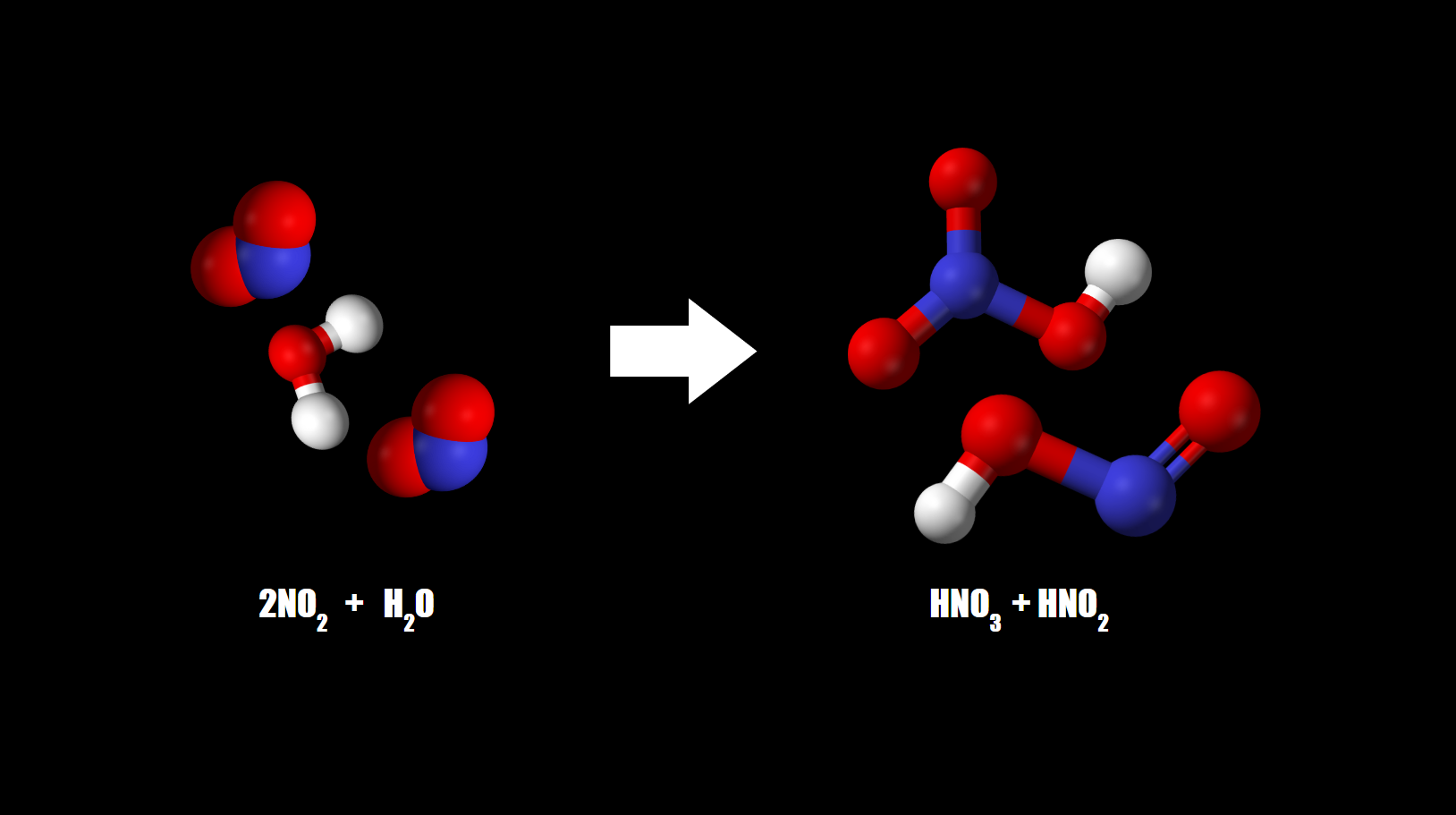
Water Droplets
A disproportionation reaction occurs between nitrogen dioxide and water when it is captured by water droplets, forming nitrite and nitrate ions.
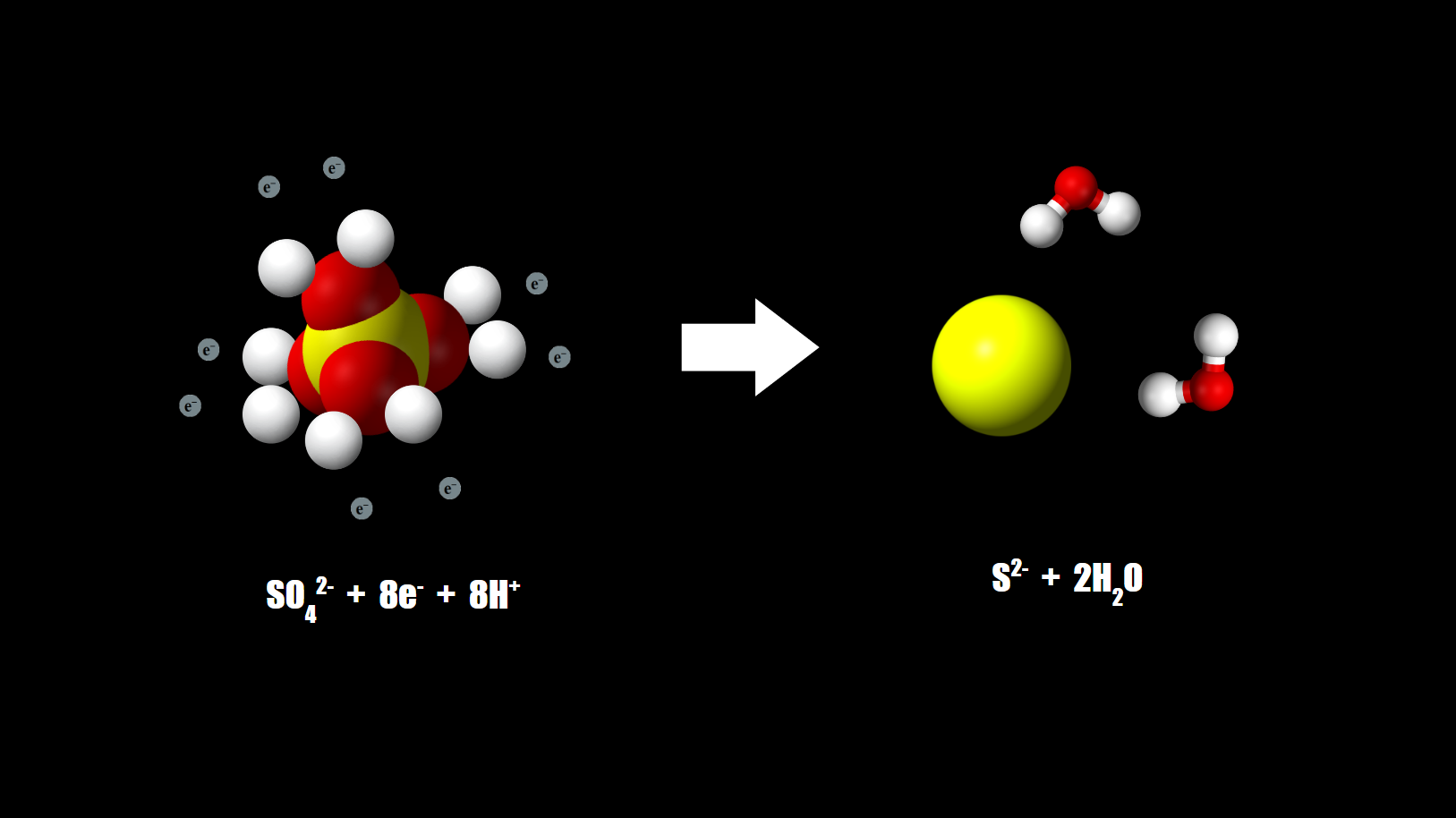
Sulfate to Sulfide
Sulfate-reducing microbes reduce sulfate to sulfide. Sulfide can be used as terminal electron acceptor.